What was the main question in your dissertation?
Unsere Fähigkeit zu sprechen und Sprache zu verwenden ist teilweise in unserer DNA verschlüsselt. Ein kleiner Defekt im Code eines einzelnen Gens kann die Ursache für die Sprachentwicklungsprobleme bei Kindern mit Sprachentwicklungsstörungen sein. Während meiner Promotion habe ich verschiedene biologische Ansätze verwendet, um zu verstehen, wie diese Gene funktionieren und wie genetische Defekte in diesen Genen unseren Sprachgebrauch stören oder verändern können.
Can you explain the (theoretical) background a bit more?
Every cell in our body contains a special code called DNA (deoxyribonucleic acid). DNA has the information to build proteins, the ‘work horses’ of the cell. Genes are groups of DNA that together form a recipe to build a protein. Humans have about 25,000 of these genes. These genes are not all active at the same time. During the early stages of human development, when embryos are forming inside their mother’s womb, different sets of genes can be active or inactive. The genes’ activation status helps the cell tune its function, shape, location, etc. For example, some cells might become brain cells, while others might become muscle cells. Many of the genes that are linked to severe developmental speech or language disorders are highly active during embryonic development, and therefore we think that they play an important role in building and shaping the brain. To study these genes, I used different methods in the lab, all based on human cells.
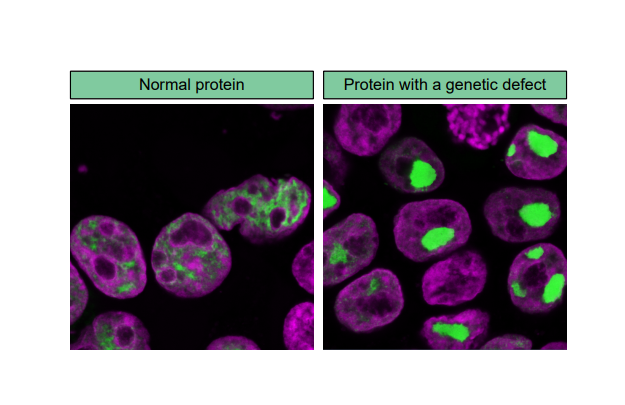
To look at the effect of many different genetic defects in a gene at the same time, I used a method that involved creating an artificial version of that gene with these different genetic defects. I inserted this artificial gene into ‘immortalized’ cells, cells that can be grown easily and indefinitely in laboratories. Then, I observed how these genetic defects affected the functions of the gene. For example, where and how the protein localizes in the cell can be crucial for what it does. In my experiments, some of the studied genetic defects changed how the protein localized to the center of the cell, i.e. the nucleus, making the protein form large aggregates that were not functional anymore (see the image below).
While the experiments with an artificial gene make it possible to look at gene functions in isolation, the real gene works together with many other genes and may have different roles depending on the moment during brain development. To investigate new gene functions and their involvement in brain development and language disorders, I grew special cells in the laboratory called “stem cells”. Stem cells are a specific type of unspecialized cells that can become any type of cell in the body. Under special lab conditions I grew these stem cells into mature brain cells. I grew cells with and without a genetic defect in a gene linked to developmental speech disorders. This helped me to understand the role of that gene during the development of brain cells.
Why is it important to answer this question?
Although we have become pretty good at reading out the full DNA code thanks to recent technological developments, it is still quite hard to interpret what small changes in the DNA actually do. By using human cell models in the lab, we can test whether such genetic changes do something to the function of the gene. With that information we can understand whether a change in the DNA can indeed be linked to a disorder, and also why certain genetic changes can have quite different disorder outcomes than other changes, sometimes even changes occurring in the same gene. This way, we can provide a better diagnosis, and adjust therapy, but it could also change where and how we look for potential treatments.
Can you tell us about one particular project?
One of the projects of my thesis started when we got contacted by collaborators from the UK, who found a child with a severe speech delay, and a genetic change in a gene that had not been associated with any disorder so far. In a large international collaboration, we looked for more people with genetic defects in that gene, and what we noticed was that there seemed to be two different groups of patients. One group had very severe symptoms, including epilepsy and sometimes even early death, while the other group had relatively mild symptoms. These two groups had different types of genetic changes, but both affected the same gene. We then went into the lab, to see if we could show that these different types of genetic defects have different effects on the function of the gene in human cells, and we could. The gene that was affected normally controls the activity of many other genes. While the genetic changes found in people with a mild syndrome stopped this control function, the changes in the severe form of the disorder made the gene actually work harder, not only changing the activity of the target genes but possibly affecting many other genes as well.
What was your most interesting finding?
I think the project I described in the previous section was a very exciting one. It was very striking to see how well the biological findings from the lab fitted with what we were observing in people with the developmental disorder. This allowed us to identify two distinct disorders associated with problems in the same gene.
What are the implications of this finding? How does this push science or society forward?
With this project we showed that if we want to better understand why genetic syndromes can sometimes have different symptoms, it is important that we study the genes closely. Where in the gene are the changes? What do they do to its function? And if they have different effects on its function, can we link that back again to differences that we observe in the symptoms of genetic syndromes?
What do you want to do next?
In the last twenty years, we have made very big steps in finding genetic causes in disorders. Many different genes have now been linked to disorders. But we are now moving beyond the idea of ‘one gene, one disorder’, as biology seems often to be way more complex than that. Human cell models in the lab will be a crucial tool to understand the importance and effects of genetic changes in human disorders. I think there is still a lot to do and investigate, and I hope to continue working in this exciting research field for a bit longer.

